
A Quality Control Record Form Sample is a standardized document used to systematically capture inspection results and quality checks during production or service processes. It helps ensure that products meet specified standards by recording measurements, defects, and corrective actions. This form is essential for maintaining consistency, traceability, and accountability in quality management systems.
Quality control record form sample for manufacturing industry
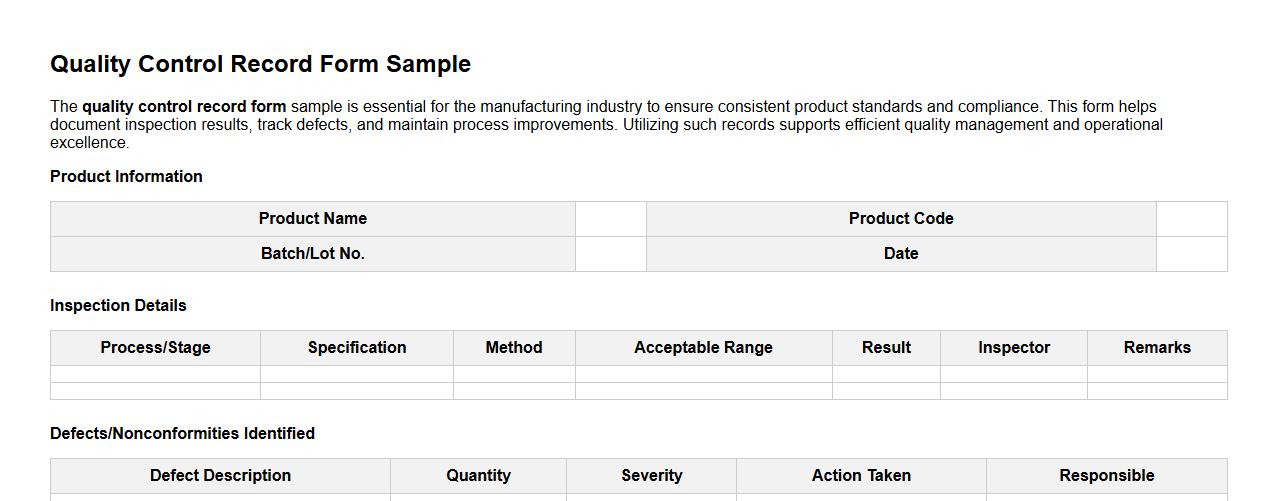
The quality control record form sample is essential for the manufacturing industry to ensure consistent product standards and compliance. This form helps document inspection results, track defects, and maintain process improvements. Utilizing such records supports efficient quality management and operational excellence.
Quality control inspection record form sample PDF
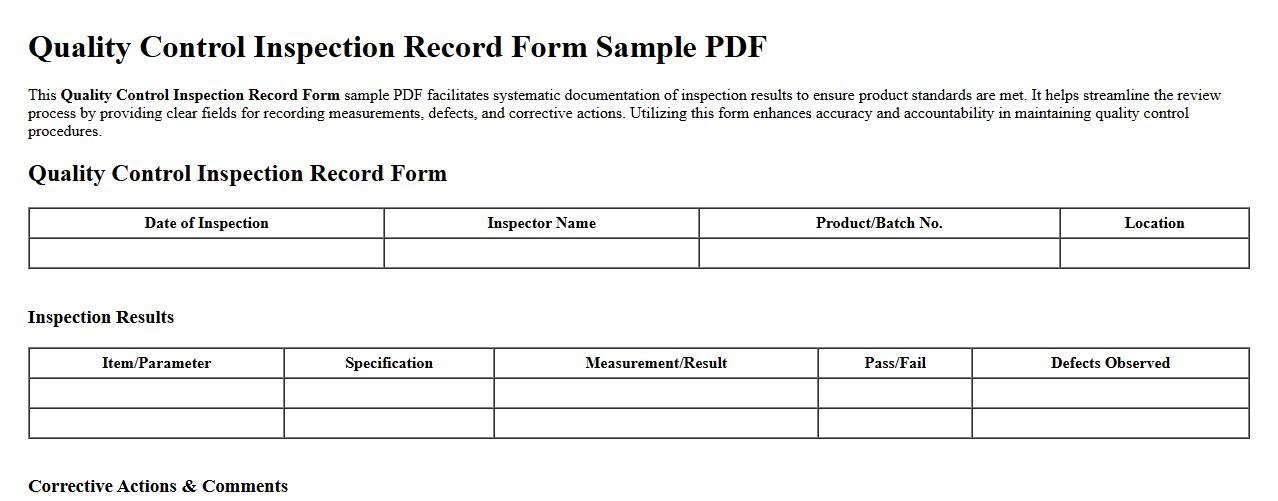
This Quality Control Inspection Record Form sample PDF facilitates systematic documentation of inspection results to ensure product standards are met. It helps streamline the review process by providing clear fields for recording measurements, defects, and corrective actions. Utilizing this form enhances accuracy and accountability in maintaining quality control procedures.
Food production quality control record form sample
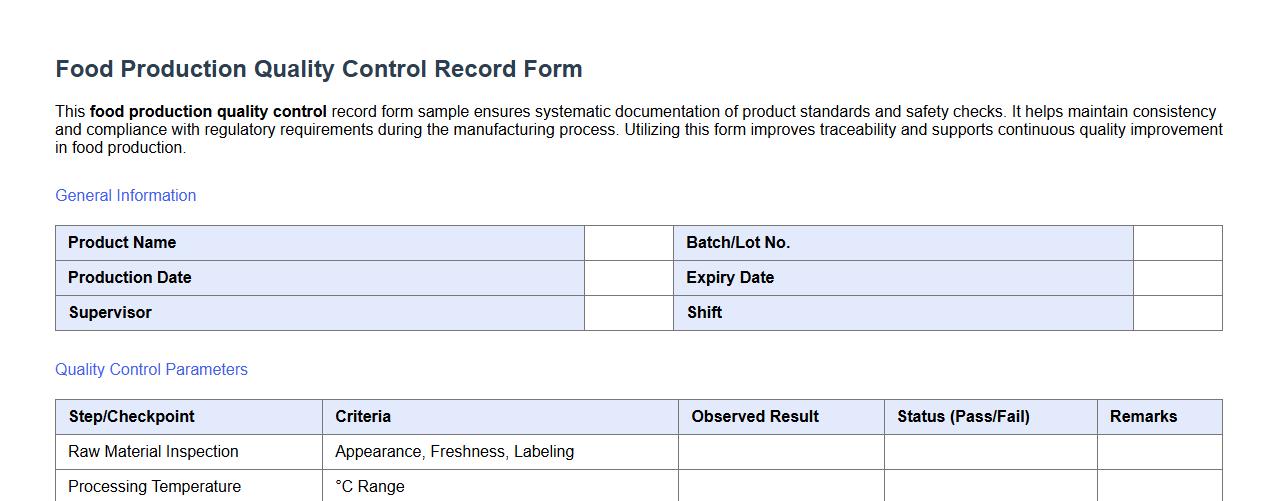
This food production quality control record form sample ensures systematic documentation of product standards and safety checks. It helps maintain consistency and compliance with regulatory requirements during the manufacturing process. Utilizing this form improves traceability and supports continuous quality improvement in food production.
Quality control record form sample for laboratory testing
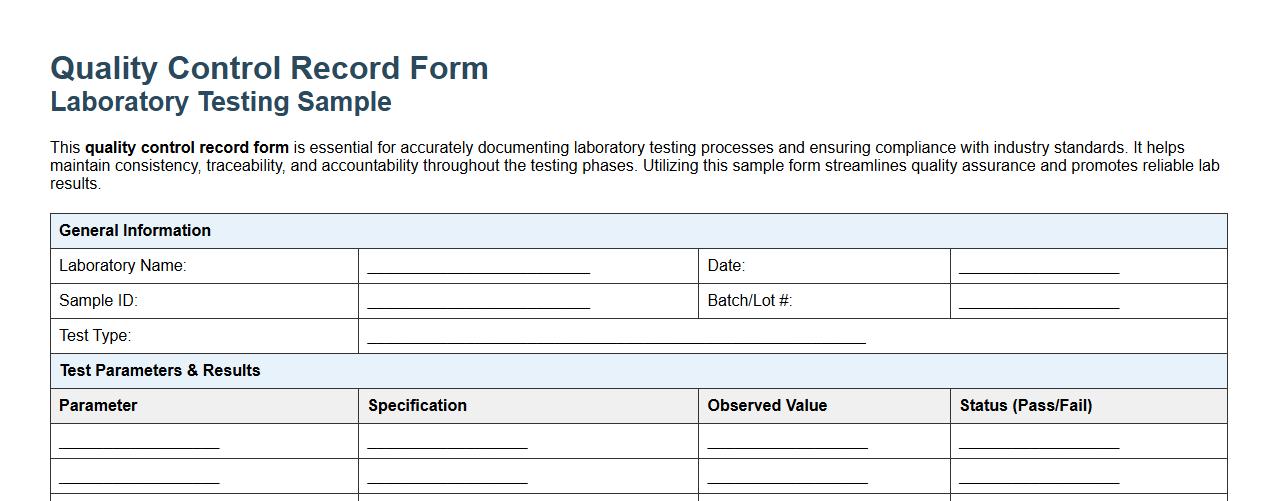
This quality control record form is essential for accurately documenting laboratory testing processes and ensuring compliance with industry standards. It helps maintain consistency, traceability, and accountability throughout the testing phases. Utilizing this sample form streamlines quality assurance and promotes reliable lab results.
Construction project quality control record form sample
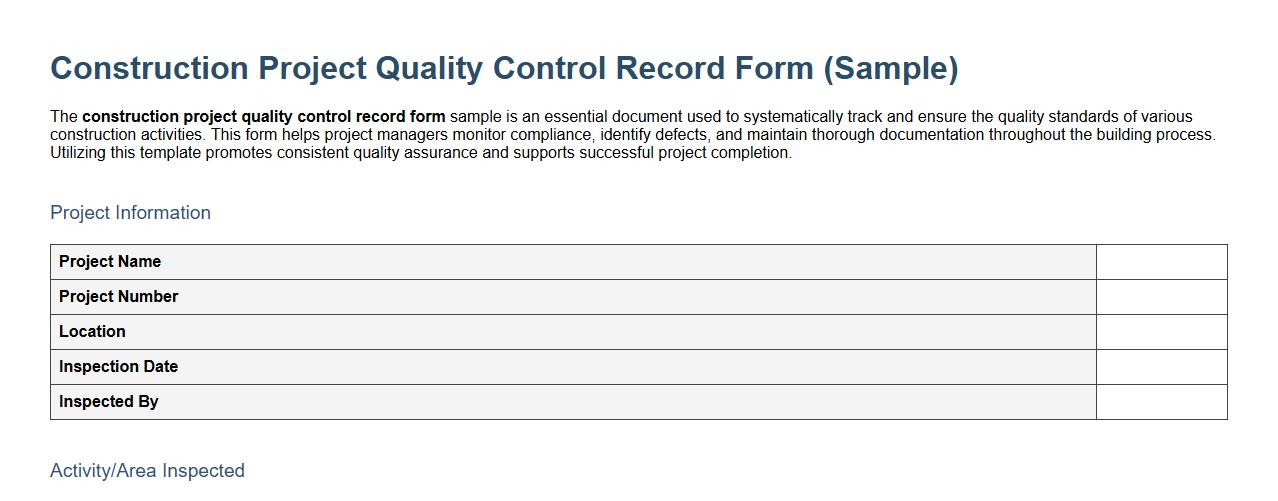
The construction project quality control record form sample is an essential document used to systematically track and ensure the quality standards of various construction activities. This form helps project managers monitor compliance, identify defects, and maintain thorough documentation throughout the building process. Utilizing this template promotes consistent quality assurance and supports successful project completion.
Sample of quality control record form for pharmaceutical industry
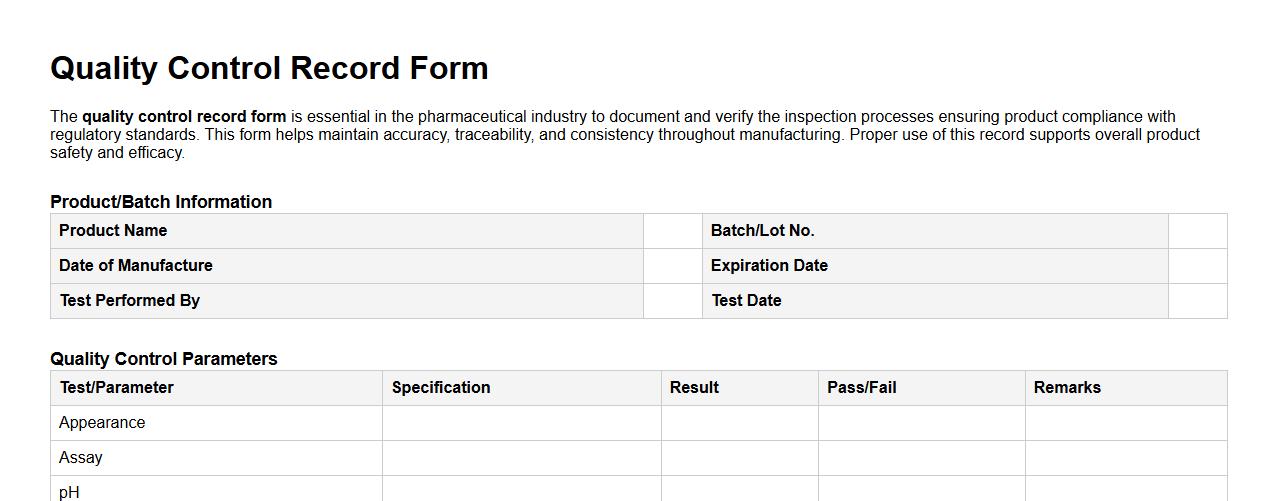
The quality control record form is essential in the pharmaceutical industry to document and verify the inspection processes ensuring product compliance with regulatory standards. This form helps maintain accuracy, traceability, and consistency throughout manufacturing. Proper use of this record supports overall product safety and efficacy.
Textile factory quality control record form sample
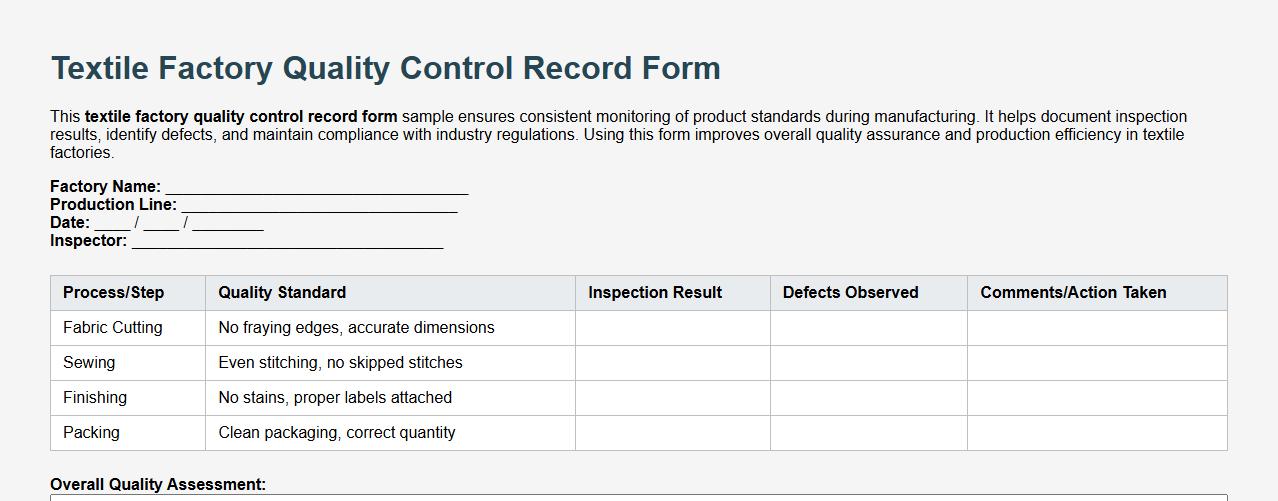
This textile factory quality control record form sample ensures consistent monitoring of product standards during manufacturing. It helps document inspection results, identify defects, and maintain compliance with industry regulations. Using this form improves overall quality assurance and production efficiency in textile factories.
Welding quality control record form sample template
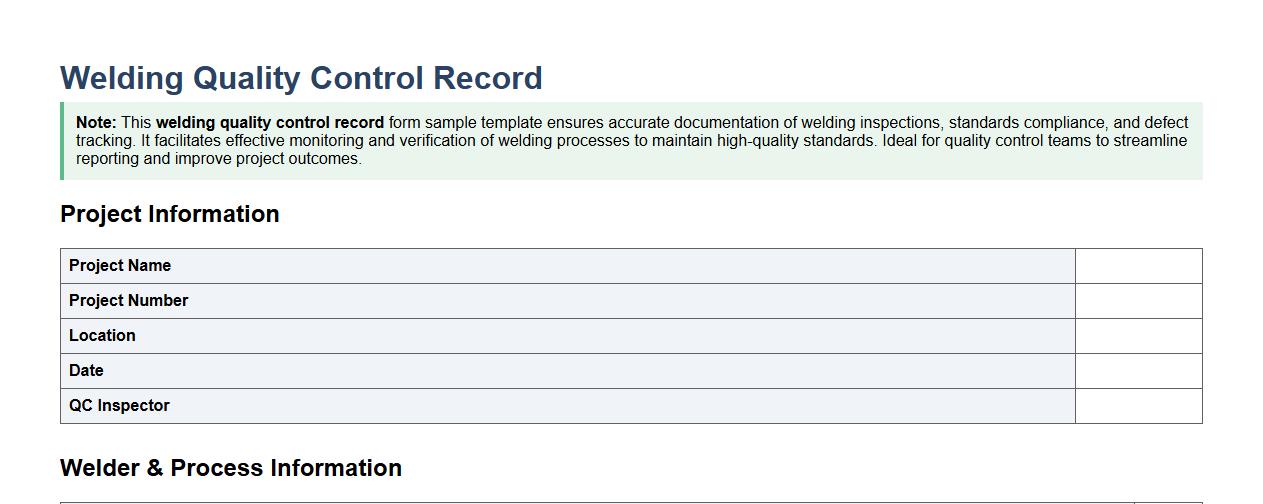
This welding quality control record form sample template ensures accurate documentation of welding inspections, standards compliance, and defect tracking. It facilitates effective monitoring and verification of welding processes to maintain high-quality standards. Ideal for quality control teams to streamline reporting and improve project outcomes.
Daily quality control record form sample for restaurants
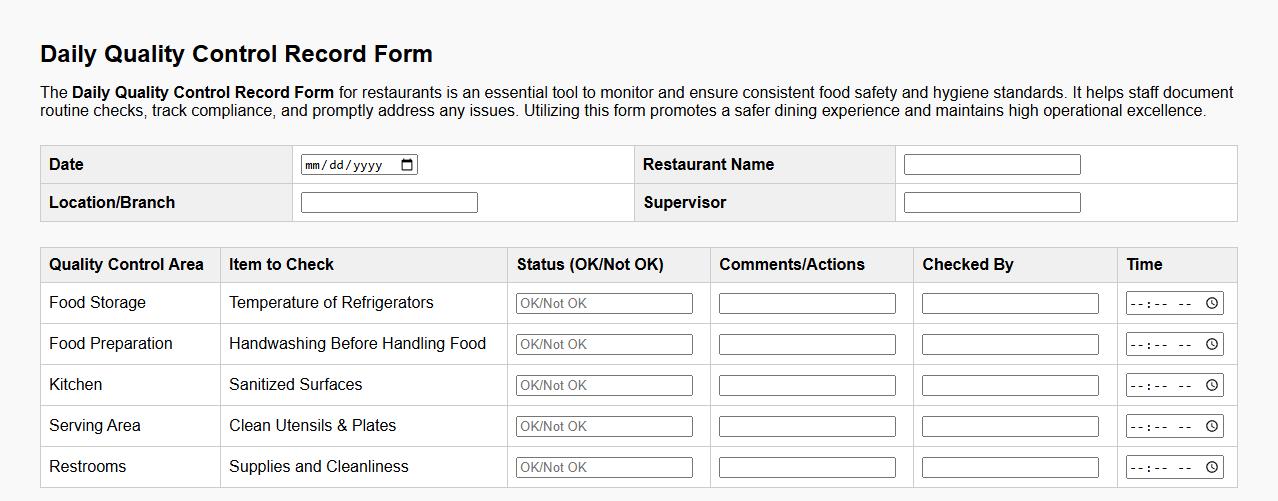
The Daily Quality Control Record Form for restaurants is an essential tool to monitor and ensure consistent food safety and hygiene standards. It helps staff document routine checks, track compliance, and promptly address any issues. Utilizing this form promotes a safer dining experience and maintains high operational excellence.
Electronic assembly quality control record form sample
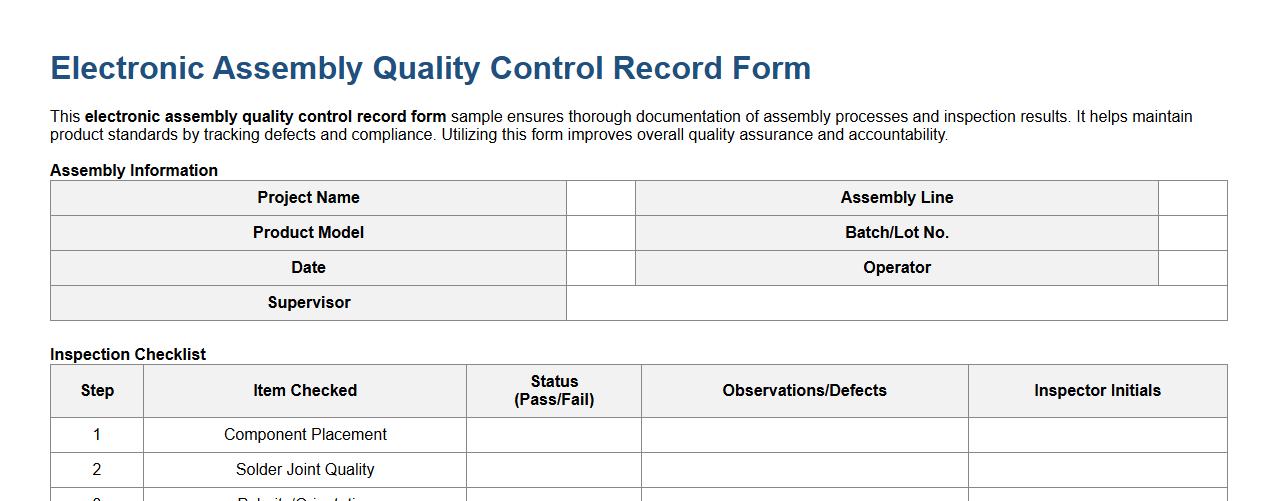
This electronic assembly quality control record form sample ensures thorough documentation of assembly processes and inspection results. It helps maintain product standards by tracking defects and compliance. Utilizing this form improves overall quality assurance and accountability.
How are nonconformities documented in the Quality Control Record Form?
Nonconformities are precisely recorded in the Quality Control Record Form to ensure traceability. Each nonconformity entry includes a detailed description, the date of identification, and the responsible personnel. This documentation facilitates effective resolution and compliance monitoring.
What metadata fields are mandatory on the QC record form?
The QC record form requires mandatory metadata fields such as product ID, inspection date, inspector name, and batch number. These fields ensure that each record is uniquely identifiable and searchable. Accurate metadata is essential for maintaining data integrity and audit readiness.
How is version control managed for Quality Control Record Forms?
Version control is managed by assigning a unique version number to each iteration of the QC record form. This system tracks updates, amendments, and historical data effectively. Maintaining version control guarantees that only the latest form is used during inspections.
What approval workflow is specified within the QC record form process?
The approval workflow involves a multi-level review where inspectors, supervisors, and quality managers must sign off on entries. This process ensures that each record is verified and authorized by the appropriate personnel. The workflow promotes accountability and compliance with quality standards.
How are corrective actions tracked using the Quality Control Record Form?
Corrective actions are tracked by linking nonconformities to specific action plans within the QC record form. The form records the status, responsible parties, and deadlines for each corrective measure. This facilitates effective monitoring and closure of quality issues.
