
A Informed Consent Form Sample provides a clear template outlining the essential information participants need before agreeing to take part in a study or medical procedure. It ensures transparency by detailing the purpose, risks, benefits, and the rights of the participant. Using a well-structured form helps protect both the participant and the organization conducting the research or treatment.
Informed consent form sample for medical research

An informed consent form sample for medical research ensures participants understand the study's purpose, procedures, risks, and benefits before agreeing to join. This document is essential for ethical compliance and protecting participants' rights. Clear and concise language helps maintain transparency throughout the research process.
Informed consent form sample for clinical trials

An informed consent form sample for clinical trials is a crucial document that ensures participants understand the study's purpose, procedures, risks, and benefits before enrolling. It promotes transparency and ethical standards by providing clear information and obtaining voluntary agreement. Using a well-structured template helps researchers maintain compliance with regulatory requirements and protect participant rights.
Informed consent form sample for minors
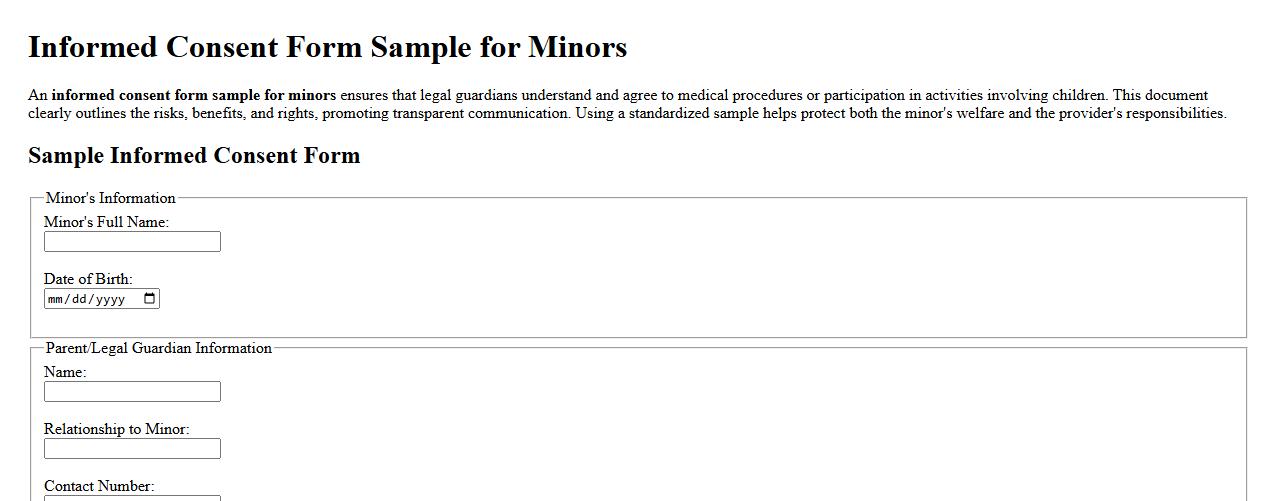
An informed consent form sample for minors ensures that legal guardians understand and agree to medical procedures or participation in activities involving children. This document clearly outlines the risks, benefits, and rights, promoting transparent communication. Using a standardized sample helps protect both the minor's welfare and the provider's responsibilities.
Informed consent form sample for psychological studies

An informed consent form sample for psychological studies ensures participants understand the study's purpose, procedures, risks, and benefits before agreeing to participate. It promotes ethical standards and protects the rights of individuals involved in research. Clear and concise information helps build trust and transparency between researchers and participants.
Informed consent form sample for surgery

An informed consent form sample for surgery ensures patients understand the procedure, associated risks, and benefits before agreeing. This document promotes transparency and supports ethical medical practices. It is essential for patient safety and legal protection.
Informed consent form sample for dental procedures

An informed consent form sample for dental procedures ensures patients understand the risks, benefits, and alternatives before treatment. This document protects both the patient and the dental provider by clearly outlining the procedure details. Utilizing a well-structured consent form promotes transparency and trust during dental care.
Informed consent form sample for data collection
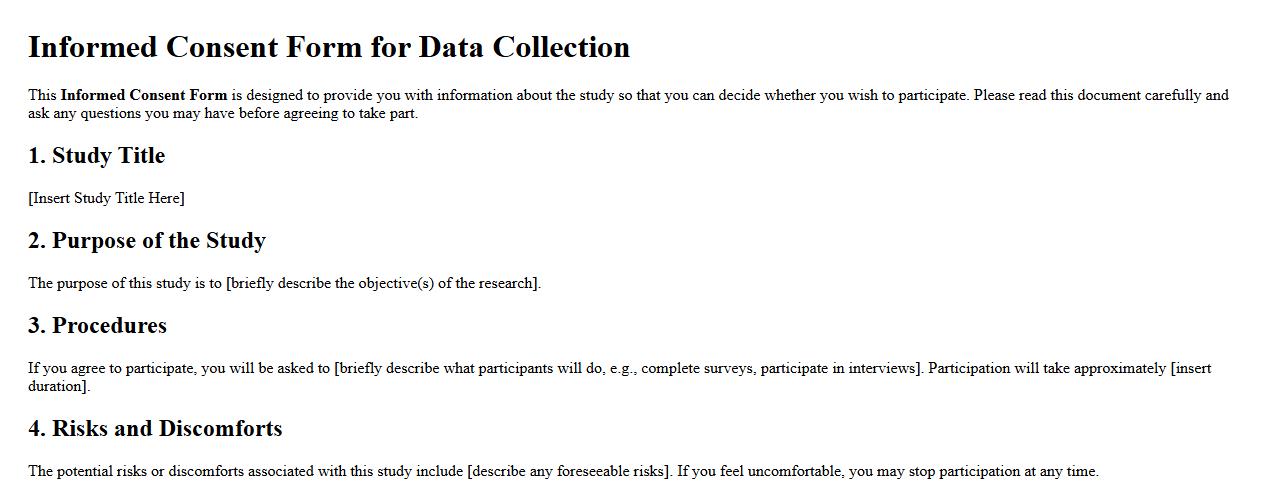
An informed consent form sample for data collection ensures participants are fully aware of the study's purpose, procedures, and potential risks before agreeing to participate. This document promotes transparency and ethical standards in research by clearly outlining participant rights and confidentiality measures. It serves as a critical tool for obtaining voluntary and informed agreement from individuals involved in data collection.
Informed consent form sample for genetic testing

An informed consent form for genetic testing ensures that individuals understand the purpose, risks, and benefits of the procedure before proceeding. This document provides clear and concise information to help patients make knowledgeable decisions about their genetic health. Properly designed consent forms uphold ethical standards and protect patient rights during genetic testing.
Informed consent form sample for telemedicine

An informed consent form sample for telemedicine ensures patients understand the nature, benefits, and risks of receiving medical care remotely. It is essential for protecting both healthcare providers and patients by clearly outlining privacy policies and treatment procedures. Using a standardized consent form helps facilitate transparent communication in virtual healthcare settings.
Informed consent form sample for educational research
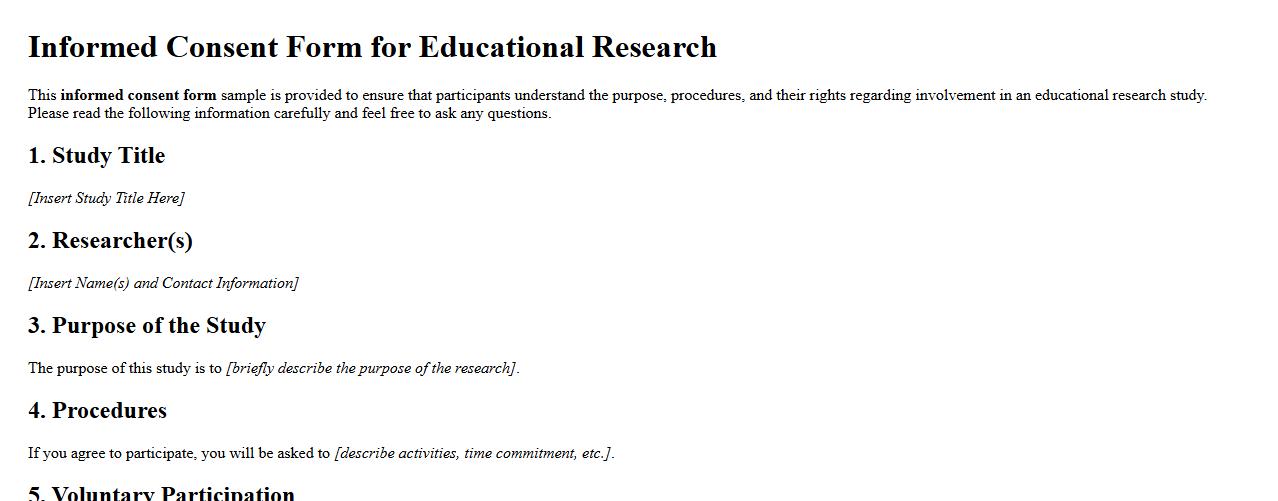
The informed consent form sample for educational research provides a clear template to ensure participants understand the study's purpose, procedures, and their rights. It emphasizes voluntary participation and confidentiality. This form is essential for ethical compliance in educational studies.
What essential elements must an Informed Consent Form include for genetic testing studies?
An Informed Consent Form for genetic testing studies must include a clear explanation of the purpose of the study, the types of genetic tests involved, and their potential outcomes. It should also detail the possible risks and benefits, including implications for family members. Additionally, information about confidentiality, data storage, and the right to withdraw consent at any time must be clearly outlined.
How can you ensure readability for non-native speakers in an Informed Consent Form?
To ensure readability for non-native speakers, the form should use simple language and avoid technical jargon wherever possible. Incorporating short sentences and logical sections with clear headings can improve understanding. Providing translations or visual aids can further assist comprehension and enhance accessibility.
What are common legal pitfalls in digital Informed Consent Forms?
Common legal pitfalls in digital Informed Consent Forms include failure to verify participant identity and inadequate capture of explicit consent. Another issue is insufficient protection of personal data, which may violate privacy regulations like GDPR or HIPAA. Finally, forms must ensure compliance with electronic signature laws to avoid disputes about consent validity.
How should risks and benefits be communicated in pediatric Informed Consent Forms?
In pediatric consent forms, risks and benefits should be communicated in a clear and age-appropriate manner to both parents and the child when appropriate. Use straightforward language focusing on potential impacts on the child's health and well-being. Additionally, emphasize the option to ask questions and the right to withdraw consent without penalty.
What specific language is recommended for data privacy in international Informed Consent Forms?
International Informed Consent Forms should include explicit language ensuring compliance with local and international data protection laws, such as GDPR. It is recommended to state clearly how data will be collected, stored, and shared, and the participant's rights regarding their data. The form should also mention safeguards for data security and the process for addressing breaches or participant requests.
